Answer:
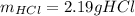
Step-by-step explanation:
Hello,
In this case, for the reaction between hydrochloric acid and sodium hydroxide, for 2.4 g of base, we can compute the neutralized grams of acid by applying the 1:1 molar ratio between them and their molar masses, 36.45 g/mol and 40 g/mol respectively as shown below by stoichiometry:
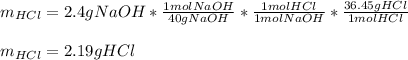
Best regards.