Step-by-step explanation:
The value of enthalpy and entropy at state 1 will be determined according to the given pressure and temperature as follows using interpolation from A-13 is as follows.
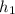 = 247.88 kJ/kg,
= 247.88 kJ/kg,
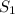 = 0.9579 kJ/kg K
= 0.9579 kJ/kg K
At state 2, isentropic enthalpy will be determined from the condition
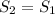 and given pressure at 2 with data from A-13 using interpolation is:
and given pressure at 2 with data from A-13 using interpolation is:
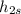 = 279.45 kJ/kg
= 279.45 kJ/kg
We will calculate actual enthalpy at state 2 using given pressure and temperature from A-13 as follows.
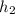 = 286.71 kJ/kg
= 286.71 kJ/kg
Hence, isentropic compressor efficiency will be calculated using standard relation as:
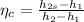
=
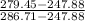
= 0.813
Now, at state 3 enthaply is determined by temperature at state 3, that is,
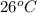 for given pressure as per saturated liquid approximation and data from A-11.
for given pressure as per saturated liquid approximation and data from A-11.
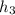 = 87.83 kJ/Kg
= 87.83 kJ/Kg
Using energy balance in 2-3, the rate of heat supplied to the heated room is as follows.
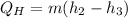
= 0.022 (286.71 - 87.83) kW
= 4.38 kW
Now, COP will be calculated using power that is expressed through energy balance in 1-2 as follows.
COP =
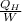
=
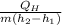
=
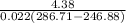
= 5.13
In an ideal vapour-compression cycle, the enthalpy and entropy at state 1 will be obtained from given pressure and state with data from A-12:
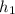 = 244.5 kJ/kg
= 244.5 kJ/kg
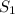 = 0.93788 kJ/kg K
= 0.93788 kJ/kg K
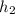 = 273.71 kJ/kg
= 273.71 kJ/kg
At state 3, enthalpy will be determined from given pressure and state with data from A-12 as follows.
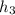 = 95.48 kJ/kg
= 95.48 kJ/kg
Hence, using energy balance in 2-3 the rate of heat supplied will be calculated as follows.
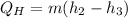
= 0.022 (273.31 - 95.48) kW
= 3.91 kW
The power input which is expressed through energy balance in 1-2 will be used to determine COP as follows.
COP =
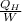
=
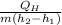
=
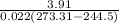
= 6.17