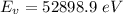Answer:
The energy of a single photon of X‑rays is
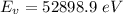
Step-by-step explanation:
From the question we are told that
The mass of the tissue is
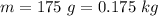
The amount of energy delivered is
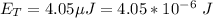
The wavelength is
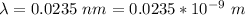
Generally the energy of a single photon is mathematically represented as
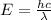
Where h is the Planck's constant with values
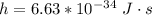
and c is the speed of light with values
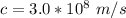
Substituting values
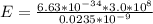
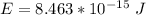
Converting this to electron volt we have
![E_v = 8.464 *10^(-15) * [(1 )/(1.6*10^(-19)) ]](https://img.qammunity.org/2021/formulas/physics/college/z41cjh81iqvioqa82y5sk39pcvegb7xept.png)