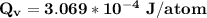Answer:
the energy vacancies for formation in silver is
Step-by-step explanation:
Given that:
the equilibrium number of vacancies at 800 °C
i.e T = 800°C is 3.6 x 10¹⁷ cm3
Atomic weight of sliver = 107.9 g/mol
Density of silver = 9.5 g/cm³
Let's first determine the number of atoms in silver
Let silver be represented by N
SO;
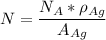
where ;
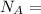 avogadro's number =
avogadro's number =
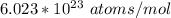
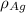 = Density of silver = 9.5 g/cm³
= Density of silver = 9.5 g/cm³
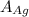 = Atomic weight of sliver = 107.9 g/mol
= Atomic weight of sliver = 107.9 g/mol
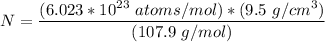
N = 5.30 × 10²⁸ atoms/m³
However;
The equation for equilibrium number of vacancies can be represented by the equation:
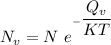
From above; Considering the natural logarithm on both sides; we have:
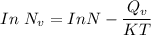
Making
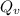 the subject of the formula; we have:
the subject of the formula; we have:
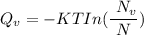
where;
K = Boltzmann constant = 8.62 × 10⁻⁵ eV/atom .K
Temperature T = 800 °C = (800+ 273) K = 1073 K
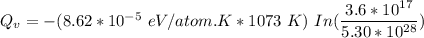
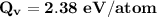
Where;
1 eV = 1.602176565 × 10⁻¹⁹ J
Then
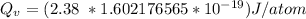
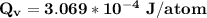
Thus, the energy vacancies for formation in silver is