The given question is incomplete. The complete question is as follows.
Steam is contained in a closed rigid container with a volume of 1 m3. Initially, the pressure and temperature of the steam are 10 bar and 500°C, respectively. The temperature drops as a result of heat transfer to the surroundings. Determine
(a) the temperature at which condensation first occurs, in
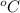 ,
,
(b) the fraction of the total mass that has condensed when the pressure reaches 0.5 bar.
(c) What is the volume, in
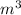 , occupied by saturated liquid at the final state?
, occupied by saturated liquid at the final state?
Step-by-step explanation:
Using the property tables
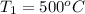 ,
,
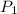 = 10 bar
= 10 bar
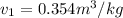
(a) During the process, specific volume remains constant.
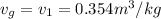
T =
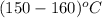
Using inter-polation we get,
T =
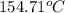
The temperature at which condensation first occurs is
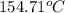 .
.
(b) When the system will reach at state 3 according to the table at 0.5 bar then
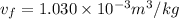
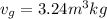
Let us assume "x" be the gravity if stream
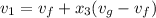
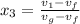
=
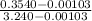
= 0.109
At state 3, the fraction of total mass condensed is as follows.
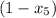 = 1 - 0.109
= 1 - 0.109
= 0.891
The fraction of the total mass that has condensed when the pressure reaches 0.5 bar is 0.891.
(c) Hence, total mass of the system is calculated as follows.
m =
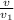
=
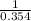
= 2.825 kg
Therefore, at final state the total volume occupied by saturated liquid is as follows.
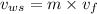
=
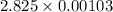
=
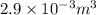
The volume occupied by saturated liquid at the final state is
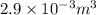 .
.