Answer:
The final temperature is T2= 5.35°C
Step-by-step explanation:
Apply the Gay-lussacs's law we have
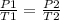
P1, initial pressure= 5.00 x 10^6 Pa
T1, initiation temperature= 25.°C
P2, final pressure= 1.07 x 10^6 Pa
T2, final temperature= ?
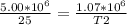
Cross multiplying and making T2 subject of formula we have
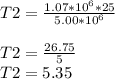
T2= 5.35°C