Answer:
see explanation
Explanation:
Given that p is inversely proportional to
![\sqrt[3]{V}](https://img.qammunity.org/2021/formulas/mathematics/middle-school/mizz38wws18nsbil2fbzzx8uot2owqypfr.png) then the equation relating them is
then the equation relating them is
p =
![\frac{k}{\sqrt[3]{V} }](https://img.qammunity.org/2021/formulas/mathematics/high-school/n5f1sbyb3i95szawi1q3a7zztepququu7d.png) ← k is the constant of proportion
← k is the constant of proportion
To find k use the condition when V = 64, p = 6 , thus
6 =
![\frac{k}{\sqrt[3]{64} }](https://img.qammunity.org/2021/formulas/mathematics/high-school/ubke6puezqaxz2919329nscxy0uoltehdn.png) =
=
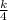 ( multiply both sides by 4 )
( multiply both sides by 4 )
24 = k
p =
![\frac{24}{\sqrt[3]{V} }](https://img.qammunity.org/2021/formulas/mathematics/high-school/xw7oucelgaqv73k7pzty79lisbxs2g8b5e.png) ← equation of proportion
← equation of proportion
(a)
When V = 27, then
p =
![\frac{24}{\sqrt[3]{27} }](https://img.qammunity.org/2021/formulas/mathematics/high-school/76x2cps1dsyigdeludlozdzi7hh68il3wt.png) =
=
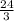 = 8
= 8
(b)
When p = 2, then
2 =
![\frac{24}{\sqrt[3]{V} }](https://img.qammunity.org/2021/formulas/mathematics/high-school/xw7oucelgaqv73k7pzty79lisbxs2g8b5e.png) ( multiply both sides by
( multiply both sides by
![\sqrt[3]{V}](https://img.qammunity.org/2021/formulas/mathematics/middle-school/mizz38wws18nsbil2fbzzx8uot2owqypfr.png) )
)
2
![\sqrt[3]{V}](https://img.qammunity.org/2021/formulas/mathematics/middle-school/mizz38wws18nsbil2fbzzx8uot2owqypfr.png) = 24 ( divide both sides by 2 )
= 24 ( divide both sides by 2 )
![\sqrt[3]{V}](https://img.qammunity.org/2021/formulas/mathematics/middle-school/mizz38wws18nsbil2fbzzx8uot2owqypfr.png) = 12 ( cube both sides )
= 12 ( cube both sides )
V = 12³ = 1728