Answer:
Therefore , 37.27g of CO₂ unreacted or remained because KO₂ is limiting
Step-by-step explanation:
The balanced chemical equation is
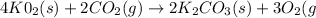
we calculate the moles of KO₂ and CO₂
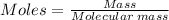
Moles of KO₂ = 25.0/71.1 = 0.3516 moles
Moles of CO₂ = 45.0/44 = 1.0227 moles
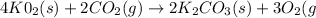
1 mole of KO₂ requires 1/2 mole of CO₂
0.3516 moles of KO₂ requires
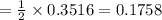
∴ no of mole of CO₂ required to complete reaction is 0.1758 moles
Avalaible no of mole of CO₂ is 1.0227 moles
∴ The exces no of mole of CO₂ is 1.0227 - 0.1758
= 0.8469 moles of CO₂
∴ mass of CO₂ that did not react is
no of mole x molar mass
=0.8469 x 44.01
= 37.27g of CO₂
Therefore , 37.27g of CO₂ unreacted or remained because KO₂ is limiting