Answer: Equilibrium constant for this reaction is
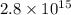 .
.
Step-by-step explanation:
Chemical reaction equation for the formation of nickel cyanide complex is as follows.
 + 2OH^(-)(aq)](https://img.qammunity.org/2021/formulas/chemistry/college/e483jl4gssiph2x7mkhd44g3ueo3r5xlfz.png)
We know that,
K =
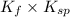
We are given that,
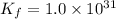
and,
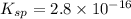
Hence, we will calculate the value of K as follows.
K =
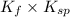
K =
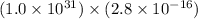
=
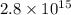
Thus, we can conclude that equilibrium constant for this reaction is
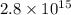 .
.