Answer:
The temperature at which the water starts boiling is 111.35 °C.
Step-by-step explanation:
First, we have to determine the total pressure experimented by water inside the piston-cylinder device. The total pressure is the sum of atmospheric and gauge pressures, the gauge pressure is the weight of piston divided by its cross-sectional area, that is:
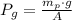
Where:
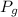 - Gauge pressure, measured in pascals.
- Gauge pressure, measured in pascals.
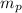 - Mass of the piston, measured in kilograms.
- Mass of the piston, measured in kilograms.
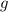 - Gravitational constant, measured in meters per square second.
- Gravitational constant, measured in meters per square second.
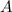 - Cross-sectional area, measured in square meters.
- Cross-sectional area, measured in square meters.
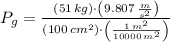
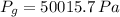
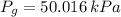
The total pressure of water is:
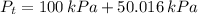
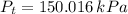
Temperature at which water starts boiling is the saturation temperature associated with total pressure, which can be obtained from property tables:
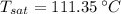
The temperature at which the water starts boiling is 111.35 °C.