Answer:
w = 252.32 N
Step-by-step explanation:
given data
balloon expand = 50 times its original size
we consider here initially pressure and volume
pressure = 645 pa
volume = 0.10 m³
solution
as in isothermala process ideal gas
PV = mRT
P =
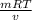
P =
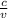
here c is constant
so work done is express as
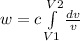
w =
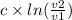
and we know c = p1 × v1
so
w = p1 × v1 ×
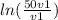
w = 645 × 0.1 × ln(50)
w = 252.32 N