Answer:
219kJ
Step-by-step explanation:
The work done (W) on a gas in an isothermal process is given by;
W = -P₁V₁ ln
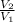 -----------------(i)
-----------------(i)
Where;
P₁ = initial pressure of the gas
V₁ = initial volume of the gas
V₂ = final volume of the gas
From the question;
P₁ = 600kPa = 6 x 10⁵Pa
V₁ = 0.45m³
V₂ = 0.2m³
Substitute these values into equation (i) as follows;
W = -6 x 10⁵ x 0.45 x ln
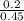
W = -6 x 10⁵ x 0.45 x ln (0.444)
W = -6 x 10⁵ x 0.45 x -0.811
W = 2.19 x 10⁵
W = 219 x 10³
W = 219kJ
Therefore, the work done on the gas during the compression process is 219kJ