Answer:
The reason is because the pressure of the air inside the room drops with time which makes opening the door to require an increased amount of force to make up for the reduced pressure inside the room
Step-by-step explanation:
From the kinetic theory of gases we have the following relation;
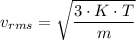
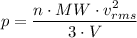
Where:
K = Boltzmann constant
T = Temperature
m = Mass
MW = Molecular weight
V = Volume
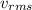 = Root mean square velocity
= Root mean square velocity
Whereby the room door is closed, the kinetic energy of the air particles will be used up such that the average velocity of the particles will decrease, given that the volume of the room is constant, the pressure inside the room will drop below the original pressure outside the room such that the force on the door due to the outside pressure is larger than the force on the door from inside the room requiring a larger amount of force to overcome the resistance of the now higher outside pressure force.