Answer:
the pressure drop is 0.21159 atm
Step-by-step explanation:
Given that:
length of the reactor L = 2.5 m
inside diameter of the reactor d= 0.025 m
diameter of alumina sphere
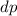 = 0.003 m
= 0.003 m
particle density = 1300 kg/m³
the bed void fraction
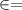 0.38
0.38
superficial mass flux m = 4684 kg/m²hr
The Feed is methane with pressure P = 5 bar and temperature T = 400 K
Density of the methane gas
 = 0.15 mol/dm ⁻³
= 0.15 mol/dm ⁻³
viscosity of methane gas
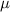 = 1.429 x 10⁻⁵ Pas
= 1.429 x 10⁻⁵ Pas
The objective is to determine the pressure drop.
Let first convert the Density of the methane gas from 0.15 mol/dm ⁻³ to kg/m³
SO; we have :
Density = 0.15 mol/dm ⁻³
Molar mass of methane gas (CH₄) = (12 + (1×4) ) = 16 mol
Density =
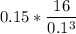
Density = 2400
Density
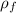 = 2.4 kg/m³
= 2.4 kg/m³
Density = mass /volume
Thus;
Volume = mass/density
Volume of the methane gas = 4684 kg/m²hr / 2.4 kg/m³
Volume of the methane gas = 1951.666 m/hr
To m/sec; we have :
Volume of the methane gas = 1951.666 * 1/3600 m/sec = 0.542130 m/sec
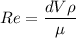
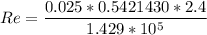
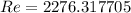
For Re > 1000
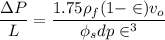
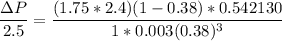
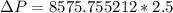
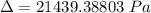
To atm ; we have
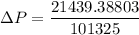
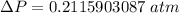
ΔP ≅ 0.21159 atm
Thus; the pressure drop is 0.21159 atm