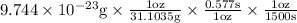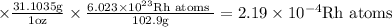Answer:
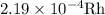
Step-by-step explanation:
Atomic mass of Nickel = 58.69 g/mol
Mass of 1 mole of nickel atom = 58.69 gm
Now, mass of 1 nickel atom = Gram atomic mass/Avogadro number
=
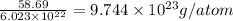
Now, price of Rhodium =$1500 per troy ounce
Price of nickel in market = 0.577$/oz
No. of rhodium atoms needed to buy 1 nickel atom is