Answer:
The dosage of the solution is 2.5 parts per million.
Step-by-step explanation:
The solvent proportion in parts per million is equal to the ratio of solution in miligrams to ratio of water in miligrams multiplied by a million. (A kilogram is equivalent to a million miligrams) That is:
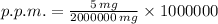
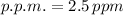
The dosage of the solution is 2.5 parts per million.