Answer:
For our assumed experiment; the expected volume of Hcl acid needed to neutralize the bicarbonate is 0.13 mL
Step-by-step explanation:
We are going attempt this question experimentally.
We know that benzoic acid originate from the relationship between benzene and a carboxylic group. So basically , the functional group of a carboxylic acid (-COOH) joins with a benzene ring(C₆H₆) to form a simple aromatic carboxylic acid known as Benzoic acid. (C₇H₆O₂)
However, it is possible to isolate benzoic acid from a bicarbonate solution in the presence of an acidified concentrated hydrochloric acid.
Let assume that ;
0.20 g of benzoic acid was reacted with 2 mL of a 20% solution of NaHCO₃, the amount of the excess NaHCO₃ can be determined by subtracting the amount of benzoic acid from the amount of NaHCO₃.
Let first calculate the number of moles in 0.20 g of benzoic acid
we know that the standard molar mass of benzoic acid is 122.12 g/mol
number of moles of benzoic acid = mass of benzoic acid/molar mass of benzoic acid =
number of moles of benzoic acid = 0.20/ 122.12
number of moles of benzoic acid = 0.0016 mol
number of moles of bicarbonate solution = mass of bicarbonate solution/ molar mass of bicarbonate solution
number of moles of bicarbonate solution = 0.2/84.00654 g/mol
number of moles of bicarbonate solution = 0.00238 mol
∴
(0.00238 - 0.0016) mol
= 7.8 × 10⁻⁴ mol
Let assume that the concentrated HCl is 12 M
Also. HCl and NaHCO₃ react together at the ratio of 1:1; thus the volume of Hcl acid needed to neutralize the bicarbonate is:
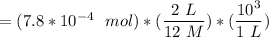
= 0.13 mL
Thus; for our assumed experiment; the expected volume of Hcl acid needed to neutralize the bicarbonate is 0.13 mL