Answer:
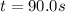
Step-by-step explanation:
Hello,
In this case, for first-order kinetics we have an integrated rate law of this reaction as:
![ln(([N_2O_5])/([N_2O_5]_0) )=-kt](https://img.qammunity.org/2021/formulas/chemistry/college/kar70fizeuenrrzvet5hp1bvpns0pry0o7.png)
Thus, we compute the time for an initial concentration of 0.280 M which ends up in 0.0476 M as shown below:
![t=(ln(([N_2O_5])/([N_2O_5]_0) ))/(-k)=(ln((0.0476M)/(0.280M)))/(-0.0197s)\\ \\t=90.0s](https://img.qammunity.org/2021/formulas/chemistry/college/zbs68txnuca9sqi0kd64yvn7upaekwp53o.png)
Best regards.