Answer: 1.29 moles of ethylene glycol must be added to 1 kg of water to make a solution with a freezing point of -2.4°C. Molality of solution is 1.29 m.
Step-by-step explanation:
Depression in freezing point is given by:
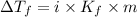
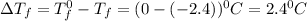 = Depression in freezing point
= Depression in freezing point
i= vant hoff factor (for non electrolyte , i = 1)
 = freezing point constant for water=
= freezing point constant for water=
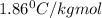
m= molality =
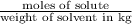
m= molality =
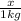
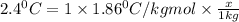
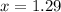
Molality =
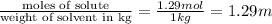
Thus 1.29 moles of ethylene glycol must be added to 1 kg of water to make a solution with a freezing point of -2.4°C. Molality of solution is 1.29 m.