Answer:
526.3g of aspitin must be added
Step-by-step explanation:
Using Raoult's law, the pressure of an ideal solution is equal to the vapour pressure of the pure solvent times mole fraction in the mixture.
The formula is:
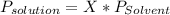
As vapour pressure of the solution you want is 458.5mmHg and the vapour pressure of the pure solvent is 463.6mmHg:
458.5mmHg = X * 463.6mmHg
0.9890 = X.
That is:
0.9890 = Moles aspirin / Moles of diethyl ether.
Moles of diethyl ether are:
219.0g × (1mol / 74.12g) = 2.955 moles of diethyl ether.
That means moles of aspirin you need are:
0.9890 = Moles aspirin / 2.955 moles.
Moles aspirin = 2.922 moles.
As molar mass of aspirin is 180.1g/mol:
2.922 moles × ( 180.1g / mol) =
526.3g of aspitin must be added