Answer:
The mass flow rate of cooling water required to cool the refrigerant is
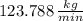 .
.
Step-by-step explanation:
A condenser is a heat exchanger used to cool working fluid (Refrigerant 134a) at the expense of cooling fluid (water), which works usually at steady state. Let suppose that there is no heat interactions between condenser and surroundings.The condenser is modelled after the First Law of Thermodynamics, which states:
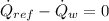
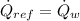
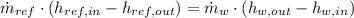
The mass flow rate of the cooling water is now cleared:
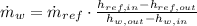
Given that
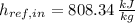 ,
,
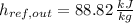 ,
,
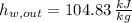 and
and
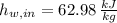 , the mass flow of the cooling water is:
, the mass flow of the cooling water is:
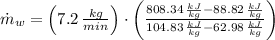

The mass flow rate of cooling water required to cool the refrigerant is
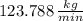 .
.