Answer:
1.95 mm
Step-by-step explanation:
Let's calculate the carbon conc. 0.194 wt% after a 6 h treatment using the expression:
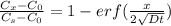
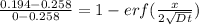
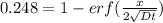
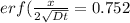
From the erf z table, at erf(z) = 0.752
z = 0.80
Thus,
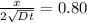
Where d =
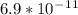
Time, t = 6 hrs = (6 * 60mins * 60secs)
Substituting values:
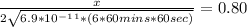
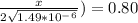
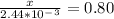
Solve for x:
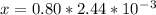
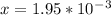
x = 1.95 mm
The position will the carbon concentration be 0.194 wt% after a 6 h treatment is at 1.95 mm