Answer:
884.8 mL
Step-by-step explanation:
If we have STP condition (Standard temperature and pressure), we will have the following relationship:
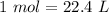 or
or
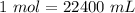 . With this in mind we can do the conversion:
. With this in mind we can do the conversion:
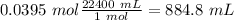
In the 0.0395 mol of fluorine gas, we will have 884.8 mL of gas at STP conditions.