Answer:
C = 4.60x10⁻⁵ M
Step-by-step explanation:
The concentration of the compound can be calculated using Beer-Lambert Law:
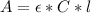
Where:
A: is the absorbance of the ethanol = 0.58
ε: is the molar absorptivity of the ethanol = 12600 M⁻¹cm⁻¹
C: is the concentration of the compound =?
l: is the optical path length = 1 cm
Hence, the concentration of the compound is:
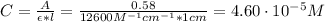
Therefore, the concentration of the compound is 4.60x10⁻⁵ M.
I hope it helps you!