Answer: V=67.2 L
Step-by-step explanation:
For this problem we will need to use the Ideal Gas Law.
Ideal Gas Law: PV=nRT
P=1.00 atm (STP)
V=?
n=3.00 mol
R=0.08206Latm/Kmol
T=273.15 K (STP)
To find V, we would manipulate the equation to V=nRT/P
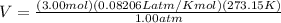
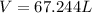
With significan figures, our answer is V=67.2 L.