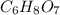Answer: The molecular formula for the given organic compound X is
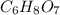
Step-by-step explanation:
We are given:
Mass of
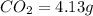
Mass of
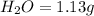
We know that:
Molar mass of carbon dioxide = 44 g/mol
Molar mass of water = 18 g/mol
For calculating the mass of carbon:
In 44g of carbon dioxide, 12 g of carbon is contained.
So, in 4.13 g of carbon dioxide, =
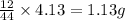 of carbon will be contained.
of carbon will be contained.
For calculating the mass of hydrogen:
In 18g of water, 2 g of hydrogen is contained.
So, in 1.13 g of water,
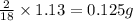 of hydrogen will be contained.
of hydrogen will be contained.
Mass of oxygen in the compound = (3.00) - (1.13+ 0.125) = 1.75 g
To formulate the empirical formula, we need to follow some steps:
Step 1: Converting the given masses into moles.
Moles of Carbon =
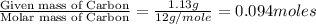
Moles of Hydrogen =
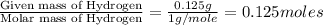
Moles of Oxygen =
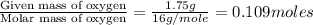
Step 2: Calculating the mole ratio of the given elements.
For the mole ratio, we divide each value of the moles by the smallest number of moles
For Carbon =
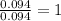
For Hydrogen =
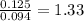
For Oxygen =
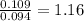
Step 3: Taking the mole ratio as their subscripts.
The ratio of C : H : O = 1: 1.33: 1.16
Converting them into whole number ratios by multiplying by 6:
The ratio of C : H : O = 6: 8: 7
Hence, the empirical formula for the given compound is
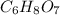
Empirical mass =
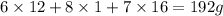
The equation used to calculate the valency is :
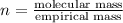
Putting values in above equation, we get:
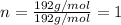
Multiplying this valency by the subscript of every element of empirical formula, we get:
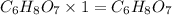
Thus molecular formula for the given organic compound X is