Answer:
Arranged in order of most soluble to least soluble in water:
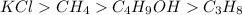
Step-by-step explanation:
Water is a polar solvent due to the electric charge it possesses. Hence it would only dissolve polar molecules.
Non-polar molecules would be dissolved by non-polar solvents.
Short chain alcohols are more soluble than butanol, but generally the polar -OH group is soluble in water.