Answer:
Approximately
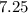 .
.
Step-by-step explanation:
If the concentration of
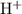 ions in a solution is
ions in a solution is
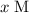 , the
, the
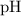 of that solution would be
of that solution would be
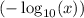 .
.
Note that the base of the logarithm in this calculation should
 . One way to be sure is to state the base explicitly. Using the change of base rule of logarithms:
. One way to be sure is to state the base explicitly. Using the change of base rule of logarithms:
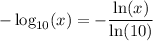 .
.
In this question, it is implied that the concentration of
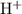 in the given solution is
in the given solution is
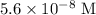 , such that
, such that
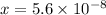 . Using the equations above:
. Using the equations above:
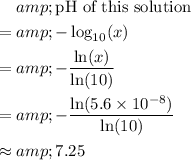 .
.