Answer:
See the answer below
Step-by-step explanation:
Aqueous solution of copper II nitrate contains solute of copper II nitrate dissolved in water. The equation for the reaction is as below:
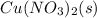 +
+
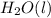 -->
-->
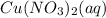
Hence, the solute chemical is copper II nitrate while the solvent is water.
Chemical formula of copper II nitrate -
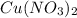
Chemical formula of water -
