Answer:
The quantity of salt at time t is
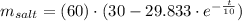 , where t is measured in minutes.
, where t is measured in minutes.
Explanation:
The law of mass conservation for control volume indicates that:
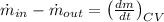
Where mass flow is the product of salt concentration and water volume flow.
The model of the tank according to the statement is:
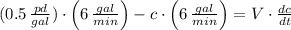
Where:
 - The salt concentration in the tank, as well at the exit of the tank, measured in
- The salt concentration in the tank, as well at the exit of the tank, measured in
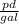 .
.
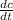 - Concentration rate of change in the tank, measured in
- Concentration rate of change in the tank, measured in
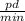 .
.
 - Volume of the tank, measured in gallons.
- Volume of the tank, measured in gallons.
The following first-order linear non-homogeneous differential equation is found:
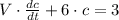
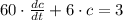
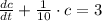
This equation is solved as follows:
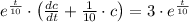
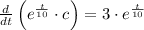
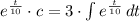
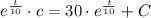
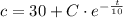
The initial concentration in the tank is:
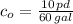
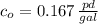
Now, the integration constant is:
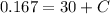
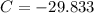
The solution of the differential equation is:
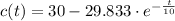
Now, the quantity of salt at time t is:
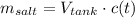
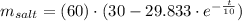
Where t is measured in minutes.