Answer:
- Four for iron, three for oxygen and 2 for iron (III) oxide:
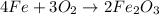
Step-by-step explanation:
Hello,
In this case, the oxidation of iron is a widely acknowledged reaction occurring in ships and other machines exposed to the air or highly oxidizing medias. Thus, by the effect of oxygen, iron undergoes oxidation typically to iron (III) oxide:
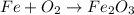
Nonetheless, the law of conservation of mass must be respected, therefore the coefficients balancing the reaction are four for iron, three for oxygen and 2 for iron (III) oxide:
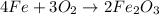
Best regards.