Answer:
1 mole of gas is contained in 22.41 liters at 101.325 kPa and 0ᴼC
Step-by-step explanation:
Ideal gases are a simplification of real gases that is done to study them more easily. It is considered to be formed by point particles, do not interact with each other and move randomly. It is also considered that the molecules of an ideal gas, in themselves, do not occupy any volume.
The pressure, P, the temperature, T, and the volume, V, of an ideal gas, are related by a simple formula called the ideal gas law:
P*V = n*R*T
where P is the gas pressure, V is the volume that occupies, T is its temperature, R is the ideal gas constant, and n is the number of moles of the gas.
In this case:
- P= 101.325 kPa= 1 atm
- V= 22.41 L
- n=?
- R= 0.082
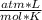
Replacing:
1 atm*22.41 L=n* 0.082
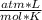 *273 K
*273 K
Solving:
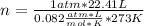
n=1 mole
1 mole of gas is contained in 22.41 liters at 101.325 kPa and 0ᴼC