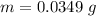Complete Question
The complete question is shown on the first uploaded image
Answer:
The mass is
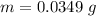
Step-by-step explanation:
From the question we are told that
The Henry's Law constant is
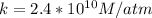
The volume of water is
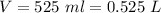
The partial pressure is
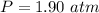
The temperature is
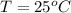
Henry's law is mathematically represented as
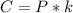
Where C is the concentration of sulfur hexafluoride(SP)
substituting value
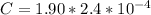
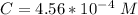
The number of moles of SP is mathematically represented as

substituting value
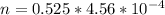
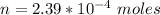
The mass of SP that dissolved is
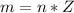
Where Z is the molar mass of SP which has a constant value of
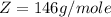
So