Answer:
Step-by-step explanation:
The equation for the react between Acetic acid and ethanol to form ethyl acetate and water is :
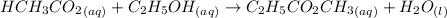
Imagine if 94.0 mmol of
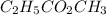 are removed from a flask; Then:
are removed from a flask; Then:
We are to answer the following questions:
1. What is the rate of the reverse reaction before any
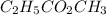 has been removed from the flask?
has been removed from the flask?
The reaction above is called an esterification reaction;
So the rate of reverse reaction before any
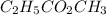 is removed is greater than zero and equal to forward reaction rate.
is removed is greater than zero and equal to forward reaction rate.
2. What is the rate of the reverse reaction just after the
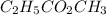 has been removed from the flask?
has been removed from the flask?
Just after the
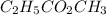 has been removed from the flask, the rate of the reverse reaction is greater than zero but less than forward reaction rate.
has been removed from the flask, the rate of the reverse reaction is greater than zero but less than forward reaction rate.
3. What is the rate of the reverse reaction when the system has again achieved equilibrium?
When the system has again achieved equilibrium, the rate of the reverse reaction is greater than zero and equal to forward reaction rate because we it has achieved the equilibrium, hence, the reaction tends to proceed in the forward direction.
4. How much less
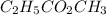 is in the flask when the system has again reached equilibrium?
is in the flask when the system has again reached equilibrium?
The
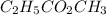 in the flask when the system has again reached equilibrium is lesser by 94.0 mmol as given right from the question
in the flask when the system has again reached equilibrium is lesser by 94.0 mmol as given right from the question