Answer:
The new pressure is 3164.58 psi.
Step-by-step explanation:
We have,
Initial pressure,
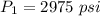
Initial temperature,
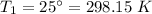
The tank is left in a car in the hot sun and the temperature increases to 44°C,
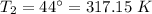
Let
 is the final pressure in the tank. Gay Lussac's law,
is the final pressure in the tank. Gay Lussac's law,
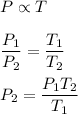
Plugging all the values,
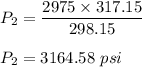
So, the new pressure is 3164.58 psi.