Answer:
final temperature = 26.5°
Step-by-step explanation:
Initial volume of water is 1 x 1 x 1 = 1

Initial temperature of water = 20° C
Density of water = 1000 kg/

volume of copper block = 0.46 x 0.46 x 0.46 = 0.097

Initial temperature of copper block = 100° C
Density of copper = 8960 kg/

Final volume of water = 1 - 0.097 = 0.903

Assumptions:
- since tank is adiabatic, there's no heat gain or loss through the walls
- the tank is perfectly full, leaving no room for cooling air
- total heat energy within the tank will be the summation of the heat energy of the copper and the water remaining in the tank.
mass of water remaining in the tank will be density x volume = 1000 x 0.903 = 903 kg
specific heat capacity of water c = 4186 J/K-kg
heat content of water left Hw = mcT = 903 x 4186 x 20 = 75.59 Mega-joules
mass of copper will be density x volume = 8960 x 0.097 = 869.12 kg
specific heat capacity of copper is 385 J/K-kg
heat content of copper Hc = mcT = 869.12 x 385 x 100 = 33.46 Mega-joules
total heat in the system = 75.59 + 33.46 = 109.05 Mega-joules
this heat will be distributed in the entire system
heat energy of water within the system = mcT
where T is the final temperature
= 903 x 4186 x T = 3779958T
for copper, heat will be
mcT = 869.12 x 385 = 334611.2T
these component heats will sum up to the final heat of the system, i.e
3779958T + 334611.2T = 109.05 x
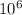
4114569.2T = 109.05 x
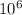
final temperature T = (109.05 x
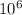 )/4114569.2 = 26.5°
)/4114569.2 = 26.5°