Answer:
a) 463.29 K
b) 8065.65 Pa
c) 0 J
Step-by-step explanation:
The parameters given are;
Volume of the tank, V = 3.72 m³
Number of moles of gas present in the tank, n = 22.1 moles
Temperature of the gas before heating, T₁ = 300 k
Heat added to the gas, ΔQ = 4.5 × 10⁴ J
Specific heat capacity at constant volume,
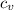 , for monatomic gas = 12.47 J/K/mole
, for monatomic gas = 12.47 J/K/mole
Avogadro's number = 6.022 × 10²³ particles per mole
a) ΔQ = n ×
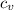 × ΔT
× ΔT
Where:
ΔT = T₂ - T₁
T₂ = Final temperature of the gas
Hence, by plugging in the values, we have;
4.5 × 10⁴ = 22.1 × 12.47 × (T₂ - 300)
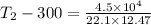
T₂ = 300 + 163.29 = 463.29 K
b) The pressure of the gas is found from the relation;
P×V = n×R×T
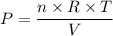
Where:
P = Pressure of the gas
R = Universal gas constant = 8.3145 J/(mol·K)
T = Temperature of the gas
V = Volume of the gas = 3.72 ³ (constant)
n = Number of moles of gas present = 22.1 moles (constant)
Hence the change in pressure is given by the relation;
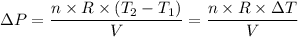
Plugging in the values, we have;
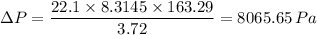
c) Work done, W, by the gas is given by the area under the pressure to volume graph which gives;
W = f(P) × ΔV
The volume given in the question is constant
∴ ΔV = 0
Hence, W = f(P) × 0 = 0 J
No work done by the gas during the process.