Answer:
M=0.816M
Step-by-step explanation:
Hello,
In this case, we should consider the following reaction:
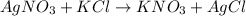
Thus, by knowing the 1:1 molar ratio of silver nitrate and potassium chloride, we can easily compute the moles of silver nitrate precipitating the 1570 mg of potassium chloride considering its molar mass of 74.5513 g/mol:
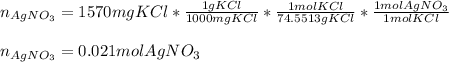
Then, by using the volume of silver nitrate in liters (0.0258 L), we can directly compute the molarity:
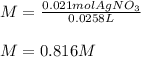
Regards.