Answer:
The final temperature of the water is 28.98 degree Celsius.
Step-by-step explanation:
It is given that,
Mass of sample of water, m = 52 grams
Initial temperature,
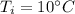
Heat absorbed,
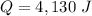
The specific heat of water is
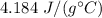
We need to find the final temperature of the water. The heat absorbed is given by the formula as follows :
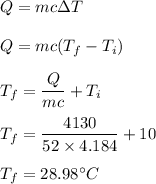
So, the final temperature of the water is 28.98 degree Celsius.