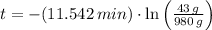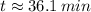Answer:
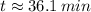
Explanation:
The time constant for the isotope decay is:
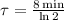
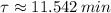
Now, the decay of the isotope is modelled after the following expression:
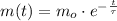
The time is now cleared with some algebraic handling:
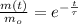
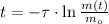
Finally, the time need for the element X to decay to 43 grams is: