Answer:
![Ka=([H^+][HCOO^-])/([HCOOH])](https://img.qammunity.org/2021/formulas/chemistry/middle-school/e95j997enbqrm0s6wlqln8cs3mymr0w68c.png)
Step-by-step explanation:
Hello,
In this case, the weak ionization reaction for formic acid is:
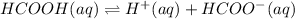
In such a way, we simply recall the law of mass action in order to represent the weak ionization constant, Ka, for such process, by taking into account that the concentration of products is divided over the concentration of reactants as shown below:
![Ka=([H^+][HCOO^-])/([HCOOH])](https://img.qammunity.org/2021/formulas/chemistry/middle-school/e95j997enbqrm0s6wlqln8cs3mymr0w68c.png)
Best regards.