Answer:
0.5 M
Step-by-step explanation:
First we have to start with the molarity equation:
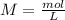
We need to know the amount of moles and the litters.
If we have 100 mL we can convert this value to “L”, so:
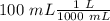
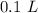
Now we can continue with the moles, for this we have to know the formula of sodium sulfate
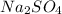 , with this formula we can calculate the molar mass if we know the atomic mass of each atom on the formula (Na: 23 g/mol, S: 32 g/mol, O: 16 g/mol). We have to multiply each atomic mass by the amount of atoms in the formula, so:
, with this formula we can calculate the molar mass if we know the atomic mass of each atom on the formula (Na: 23 g/mol, S: 32 g/mol, O: 16 g/mol). We have to multiply each atomic mass by the amount of atoms in the formula, so:
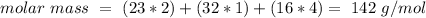
In other words:
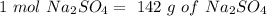
Now we can calculate the moles:
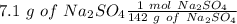
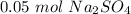
Finally, we can calculate the molarity:
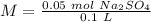
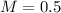
I hope it helps!