Answer:
Hydrolysis= Addition of a water to a molecule to produce two molecules
Neutralization= Reaction between and acid and a base to produce a salt and water.
Step-by-step explanation:
These are two common reactions but they are very different. Lets see a few examples:
In a hydrolysis we add a water molecule to produce several substances (see figure 1).
In this reaction water (
 ) is added in order to "break" the molecule and produce several subtances.
) is added in order to "break" the molecule and produce several subtances.
When we have a neutralization reaction, an acid (a subtance that can produce hydronium ions
 ) reacts with a base (a subtance that can produce hydronium ions
) reacts with a base (a subtance that can produce hydronium ions
 ) to produce water an a salt:
) to produce water an a salt:
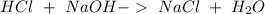
In this reaction we an acid (
 ) and a base (
) and a base (
 ) and this reaction give a salt (
) and this reaction give a salt (
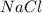 ) and water (
) and water (
 ).
).
I hope it helps!