Answer: The molarity of the diluted solution be 0.10 M
Step-by-step explanation:
According to the dilution law,
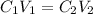
where,
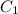 = concentration of concentrated solution = 0.15 M
= concentration of concentrated solution = 0.15 M
 = volume of concentrated solution = 100 ml
= volume of concentrated solution = 100 ml
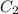 = concentration of diluted solution= ?
= concentration of diluted solution= ?
 = volume of diluted solution= 150 ml
= volume of diluted solution= 150 ml
Putting the values we get:
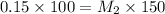
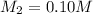
Thus the molarity of the diluted solution be 0.10 M