Answer:
About 0.151 M.
Step-by-step explanation:
Because 32.20 mL of 0.250 M NaOH was used, determine the number of moles of NaOH consumed:
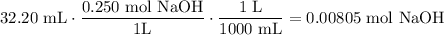
Find the number of moles of sulfuric acid reacted with using reaction stoichiometry:
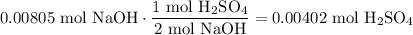
Therefore, the molarity of the original sulfuric acid solution is:
![\displaystyle \ [\text{H$_2$SO$_4$}] = \frac{0.00402\text{ mol}}{26.60\text{ mL}} \cdot \frac{1000\text{ mL}}{1\text{ L}} = 0.151\text{ M}](https://img.qammunity.org/2023/formulas/chemistry/high-school/cuuz3pq8x8rqld1wyn156nq8lqrr172j2r.png)
In conclusion, the molarity of the original sulfuric acid solution was about 0.151 M.