Answer: 0.024 ml of hydrogen gas are collected at
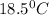 and 755.5mmHg in this single replacement reaction
and 755.5mmHg in this single replacement reaction
Step-by-step explanation:
To calculate the moles :
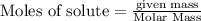
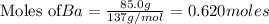
To calculate the number of moles for given molarity, we use the equation:
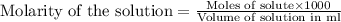
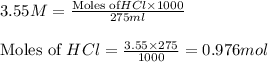
The balanced chemical reaction is:
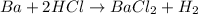
According to stoichiometry :
2 moles of
 require = 1 mole of
require = 1 mole of
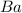
Thus 0.976 moles of
 will require=
will require=
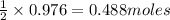 of
of
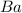
Thus
 is the limiting reagent as it limits the formation of product and
is the limiting reagent as it limits the formation of product and
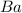 is the excess reagent.
is the excess reagent.
As 2 moles of
 give = 2 moles of
give = 2 moles of

Thus 0.976 moles of
 give =
give =
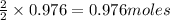 of
of

According to ideal gas equation:

P = pressure of gas = 755.5 mmHg = 0.994 atm (760 mm Hg = 1 atm )
V = Volume of gas in L = ?
n = number of moles = 0.976
R = gas constant =
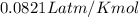
T =temperature =
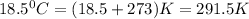
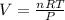
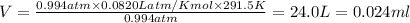 (1L=1000ml)
(1L=1000ml)
Thus 0.024 ml of hydrogen gas are collected at
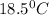 and 755.5mmHg in this single replacement reaction
and 755.5mmHg in this single replacement reaction