Answer:
Option C
Step-by-step explanation:
A double displacement reaction is the one in which two chemical compounds having a cation and anion in each exchange their positive and negative ions thereby forming new compounds or products.
Here,
The first compound
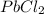 has a positive ion
has a positive ion
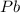 and negative ion
and negative ion

Like wise the second compound
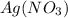 has a positive ion
has a positive ion
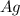 and negative ion
and negative ion
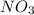
The new compounds will be formed when cation of one compound attaches with the anion of other compound. Hence the new compounds are
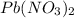
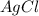
This is example of double displacement reaction.
Option C is correct