Answer:
7.15
Step-by-step explanation:
Firstly, the COP of such heat pump must be measured that is,
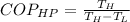
Therefore, the temperature relationship,
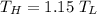
Then, we should apply the values in the COP.
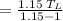
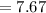
The number of heat rejected by the heat pump must then be calculated.
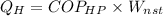
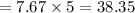
We must then calculate the refrigerant mass flow rate.
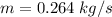
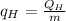
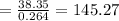
The
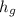 value is 145.27 and therefore the hot reservoir temperature is 64° C.
value is 145.27 and therefore the hot reservoir temperature is 64° C.
The pressure at 64 ° C is thus 1849.36 kPa by interpolation.
And, the lowest reservoir temperature must be calculated.
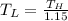

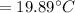
the lowest reservoir temperature = 258.703 kpa
So, the pressure ratio should be = 7.15