Answer:
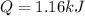
Step-by-step explanation:
Hello,
in this case, the required heat to increase the water by 5.53 °C is computed by using the mass, heat capacity and change in temperature during the process:
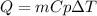
Thus, for the given data we compute it in kJ:
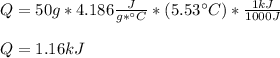
Best regards.