Answer:
The molar mass of H₂O₂ (the solute) in the aqueous solution of
H₂O₂ is 51 g
Step-by-step explanation:
Given;
H₂O₂ compound
Concentration of aqueous solution of H₂O₂ = 1.5M
The molecular mass of H₂O₂ = (1 x 2) + (16 x 2) = 34 g/mol
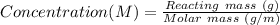
Reacting mass (g) = Concentration x Molar mass
Reacting mass (g) = 1.5 x 34
Reacting mass (g) = 51 g
Therefore, the molar mass of H₂O₂ (the solute) in the aqueous solution of
H₂O₂ is 51 g